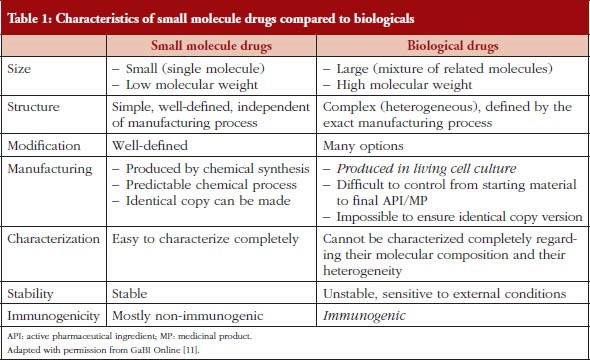
In the pharmaceutical industry, the development processes for small molecules, biologics, and dosage forms are all uniquely complex. Still, they share a common need for careful planning, rigorous design, and continuous improvement.
The development process for small molecule drugs and biologics is drastically different, presenting challenges for manufacturers, regulatory organizations, healthcare providers, and patients.
Small molecules are low molecular weight organic compounds often used in medications. The development process for small molecules is multifaceted and requires scientific and technical expertise and a robust process design strategy. Each small molecule drug, whether it’s Imatinib, Remdesivir, or Tenofovir Disoproxil Fumarate (TDF), presents a unique set of challenges that must be overcome to commercialize the drug successfully. The therapeutic potential of these small molecules can be realized through robust design strategies and continuous improvement.
Biologics, on the other hand, are complex, large-molecule drugs that are typically produced using living cells. The process development for biologics also requires careful planning, technical expertise, and a robust process design strategy. The biologics Humira, Herceptin, and insulin highlight the importance of robust process development in bringing these life-changing therapies to patients. As we continue to advance our understanding of biological systems and bioprocessing technologies, we are better equipped to navigate the complex pathway of biologics process development.
Finally, dosage forms refer to the physical form of a chemical compound or biological dose used as a drug or medication, such as tablets, capsules, or solutions. Developing different dosage forms is crucial to ensuring patients can effectively and safely use the drug. Drugs like Metformin HCL ER, Remicade, and Tretinoin each demonstrate the importance of meticulous process development for different dosage forms. The goal is to deliver the drug effectively and safely to the site of action, and this is achieved through robust process development strategies and the continuous advancement of pharmaceutical technologies.
Comprehensive Understanding of the Product Type, the Process, and the Regulatory Landscape
Designing robust processes during CMC drug development is not a simple task. It requires a comprehensive understanding of the product, the process, and the regulatory landscape. Adopting QbD, effective risk management, thorough process validation, and a commitment to continuous improvement can significantly enhance the process’s robustness. By doing so, pharmaceutical companies can ensure that their products are safe, effective, and high-quality, benefiting patients worldwide.
The US Food and Drug Administration (FDA) encourages risk-based approaches and the adoption of QbD principles in drug product development, manufacturing, and regulation. FDA’s emphasis on QbD began with the recognition that increased testing does not necessarily improve product quality. Quality must be built into the product.
Over the years, pharmaceutical QbD has evolved with the issuance of ICH Q8 (R2) (Pharmaceutical Development), ICH Q9 (Quality Risk Management), and ICH Q10 (Pharmaceutical Quality System) In addition, the ICH Q1WG on Q8, Q9, and Q10 Questions and Answers; the ICH Q8/Q9/Q10 Points to Consider document; and ICH Q11 (Development and Manufacture of Drug Substance) have been issued, as have the conclusions of FDA-EMA’s parallel assessment of Quality-By-Design elements of marketing applications. These documents provide high-level directions for the scope and definition of QbD as it applies to the pharmaceutical industry.
Remember, the path to drug development success is paved with well-planned, meticulously executed, and continuously monitored processes. Embrace the challenge and make the most of the opportunities that robust CMC process design provides.
Quality by Design (QbD) Like…
QbD involves identifying critical quality attributes (CQAs), and process parameters and understanding how these elements affect the final product’s quality. Using the design of experiments (DoE) can help explore these relationships and optimize the process.
Pharmaceutical QbD is a systematic approach to development that begins with predefined objectives and emphasizes product and process understanding and control based on sound science and quality risk management (3). The goals of pharmaceutical QbD may include the following:
- To achieve meaningful product quality specifications that are based on clinical performance
- To increase process capability and reduce product variability and defects by enhancing product and process design, understanding, and control
- To increase product development and manufacturing efficiencies
- To enhance root cause analysis and postapproval change management
Risk Management
Risk management is an integral part of the CMC process design. It involves identifying potential risks, assessing their impact and likelihood, and implementing mitigation measures.
ICH Q9 provides a valuable framework for risk management in pharmaceutical development. It encourages applying risk-based approaches at every stage, from early product development to commercial manufacturing.
Process Validation
Process validation is a critical step in the CMC development process. It involves demonstrating that the process consistently produces a product that meets its predetermined specifications and quality attributes.
The FDA’s guidance on process validation outlines a lifecycle approach. It entails process design, process qualification, and continued process verification. It’s important to note that validation is not a one-time event but a continuous process that requires monitoring and control.
Continuous Improvement
Continuous improvement is essential for maintaining process robustness. This approach involves regularly monitoring the process, identifying opportunities for improvement, implementing changes, and evaluating their impact.
Advanced statistical techniques and process analytical technology (PAT) can be invaluable. They provide real-time data for process control and decision-making.
Small Molecule Process Development: Driving Pharmaceutical Innovation with Robust Designs
Small molecules play a crucial role in pharmaceutical innovation. They represent the majority of marketed drugs and continue to be a significant focus of research and development efforts. These low molecular weight compounds can penetrate cell membranes, interact with numerous biological targets, and are typically easier to manufacture and formulate than biologics. Therefore, they offer a broad range of therapeutic possibilities.
However, developing a robust process for small-molecule drugs is challenging. It involves several steps, including discovery, process development, and scale-up to commercial production. Each step requires careful planning, execution, and control to ensure product quality and process efficiency. Let’s delve into the specifics of small molecule process development and provide real-life examples of its successful implementation.
Discovery and Early Development
The journey of small molecule drug development begins with discovery and early development. At this stage, chemists synthesize numerous molecules that can potentially treat a specific disease.
For instance, the discovery of Imatinib, a small molecule tyrosine kinase inhibitor used to treat chronic myeloid leukemia, exemplifies the power of targeted small molecule therapeutics. Researchers designed Imatinib to inhibit the BCR-ABL tyrosine kinase, a protein that promotes cancer cell growth, providing a more precise treatment option for patients.
Process Development
Once a promising molecule is identified, the next step is process development. This involves optimizing the synthesis route and manufacturing process to ensure the drug can be produced consistently and cost-effectively at a larger scale.
A prime example of successful process development is the creation of the antiviral drug Remdesivir. Initially developed for Ebola, Remdesivir was repurposed and used as a treatment for COVID-19. The original synthesis route involved many steps and low-yielding reactions. However, through extensive process development efforts, scientists streamlined the synthesis, ultimately enabling the rapid scale-up and distribution of the drug during the pandemic.
Scale-Up and Commercial Production
The final step in small molecule process development is scale-up and commercial production. This step involves transferring the optimized process to a larger scale and validating its performance.
A recent case highlighting successful scale-up is the manufacturing of the HIV drug Tenofovir Disoproxil Fumarate (TDF). Initially, the production of TDF was complex and costly. However, through process optimization and careful scale-up, manufacturers were able to reduce the cost significantly. This achievement has made TDF more accessible to patients worldwide, particularly in low-income countries.
Biologics Process Development: Navigating the Complex Pathway from Lab to Market
Biologics represent a significant part of modern therapeutic agents, with monoclonal antibodies (mAbs) and therapeutic proteins leading the way. Unlike small molecules, biologics are large, complex molecules produced in living systems such as bacteria, yeast, or mammalian cells. They offer targeted and potent treatment options for a range of diseases, including cancer, autoimmune disorders, and infectious diseases. However, their complex nature also introduces unique challenges in process development. Here, we’ll explore the specifics of biologics process development and provide some real-life examples of its successful implementation.
Discovery and Early Development
The journey of biologics begins with discovery and early development, where scientists use techniques like phage display or hybridoma technology to generate mAbs, or recombinant DNA technology to produce therapeutic proteins.
For instance, Humira (Adalimumab), a mAb used to treat several autoimmune diseases, was developed using phage display technology. This method involves using bacteriophages to create a vast library of antibody fragments, selecting those that bind to a specific target, and then further engineering them into a full-length antibody.
Process Development
Once a promising biologic is identified, the next step is process development. This stage involves optimizing the cell culture conditions, media, and purification process to ensure a robust and scalable manufacturing process.
A case in point is the development of the mAb drug Herceptin (Trastuzumab) for treating HER2-positive breast cancer. The production of Herceptin involves culturing mammalian cells that produce the antibody, followed by a series of purification steps. The process development stage was crucial in ensuring that Herceptin could be delivered consistently and at a scale sufficient to meet patient needs.
Scale-Up and Commercial Production
The final step in biologics process development is scale-up and commercial production. This step involves transferring the optimized process to larger bioreactors, ensuring consistency across batches, and validating the approach.
The production of the protein-based drug insulin provides an excellent example of a successful scale-up. Initially extracted from the animal pancreas, insulin is produced using recombinant DNA technology in bacteria or yeast, allowing for large-scale production. The scale-up process involved significant optimization to ensure the final product’s purity and potency were maintained at larger volumes.
Dosage Form Process Development: Building the Bridge from Active Ingredient to Patient Use
Creating an appropriate dosage form is one of the most crucial aspects of drug development. The dosage form, whether a solid oral tablet, a parenteral injection, a topical cream, or an inhalable aerosol, is vital in determining how the drug is absorbed and distributed within the body. Consequently, the process development for these dosage forms is an intricate blend of science, technology, and engineering. Let’s explore the specifics of various dosage forms’ process development and provide real-life examples of their successful implementation.
Solid Oral Dosage Forms
Solid oral dosage forms, such as tablets and capsules, are the most commonly used due to their ease of administration, accurate dosing, and patient compliance. The development of these dosage forms includes blending, granulation, drying, milling, compression (for tablets), or filling (for capsules).
A well-known example is the development of extended-release tablets, like Metformin HCL ER, used to treat type 2 diabetes. The process development for this dosage form involved designing a tablet matrix that allows the drug to be slowly released over several hours, ensuring a consistent blood sugar control level.
Parenteral Dosage Forms
Parenteral dosage forms, such as injectables, are used when a rapid onset of action is required, or the drug is not absorbable or stable in the gastrointestinal tract. The process development for these dosage forms includes solubilization, sterilization, and aseptic filling.
A notable example is developing the parenteral dosage form for the mAb drug Remicade (Infliximab). The drug is delivered via intravenous infusion, and the process development involved formulating the drug as a lyophilized powder that could be reconstituted with sterile water before administration.
Topical Dosage Forms
Topical dosage forms, like creams and ointments, are used for localized drug delivery. The process development for these dosage forms involves carefully blending the drug with various excipients to create a product with the right consistency, spreadability, and drug release characteristics.
A well-known example is the development of the topical dosage form for the acne treatment drug, Tretinoin. The process development involved formulating a cream that could deliver the drug effects to the skin without causing excessive irritation.
Conclusion
Whether it’s small molecules or biologics, solid oral dosage forms or parenteral injections, the drug development journey is a complex and multifaceted endeavor. The process development phase plays a critical role in this journey, acting as the bridge between the initial discovery of a therapeutic agent and its final delivery to patients in a safe, effective, and accessible format.
In conclusion, the path of drug development is challenging but rewarding. With a robust process design strategy and a commitment to quality and continuous improvement, we can unlock the vast potential of therapeutic agents, bringing hope and healing to patients worldwide. The future of drug development is bright, full of promise and opportunities, and process development will continue to be a cornerstone of this fascinating journey.